According to Acorn AI, one of the industry’s leading platforms supporting clinical trials, in 2020, approximately 75% of new drug applications (NDA) and Biologic License Applications already incorporate real-world data. Since then, there has been a notable increase in the utilization of RWD. However, like with any pioneering advancement, there exists challenges between the potential of RWD analysis, its quality and relevancy. Additionally, researchers are required to prioritize privacy protection, address potential biases, and produce significant outcomes when working with RWD.
The growing accessibility of real-world data and real-world evidence has motivated policymakers at the U.S. Food and Drug Administration and stakeholders in the biomedical community to investigate how these resources can be effectively incorporated into the process of drug development and regulatory evaluation. Recent legislative initiatives like the 21st Century Cures Act and the sixth Prescription Drug User Fee Act (PDUFA VI) aim to address this matter by identifying key areas where the FDA should explore the potential use of RWE. These areas include supporting new uses for already approved drugs and fulfilling post-approval study requirements.
In this article, we will unlock 4 essential factors in determining and improving the overall relevancy and quality of your data for ‘fit for regulatory purposes.’
Key Terms:
Real-world data (RWD) refers to data that is regularly collected and is relevant to the health status of patients and the provision of healthcare. Examples of RWD include electronic health records, insurance claims data, as well as socio-economic, environmental, and other emerging data types.
Real-world evidence (RWE) is usually obtained from real-world data by using research methods. RWE is valuable for understanding the health status of individuals and assessing the effectiveness and safety of treatments in real-world settings. Furthermore, it provides insights that may not be easily captured by traditional research methods due to time constraints, limitations in natural settings, or the inclusion of relevant populations.
The Framework:
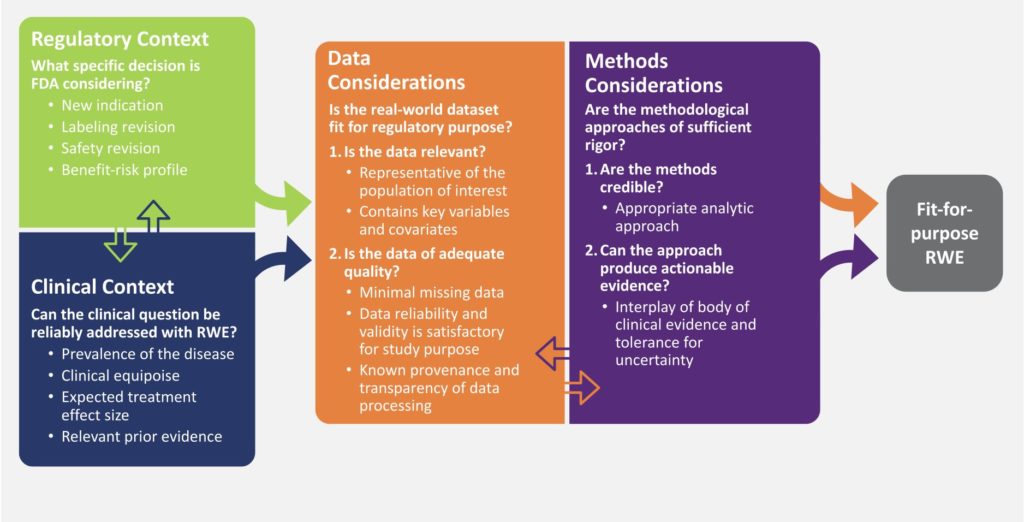
In 2017, the Duke-Margolis Center for Health Policy, the FDA and a panel of expert stakeholders, released a framework that discussed the essential factors to be taken into account when creating RWE that is suitable for regulatory use.
[Image source: https://healthpolicy.duke.edu/sites/default/files/2020-03/characterizing_rwd.pdf ]
The framework suggests that the creation of RWE fit for regulatory purposes should be influenced by four specific factors: the specific regulatory query being pursued by a sponsor, the clinical setting in which the RWE is being produced, the accessibility of relevant and high-quality real-world data (RWD), and the utilization of reliable methodologies to transform RWD into actionable evidence.
‘Fit for Regulatory Purpose’ RWD Explained
Assessing whether a real-world dataset is ‘fit for regulatory purposes’ involves considering its specific characteristics, which ultimately determine the meaning and level of confidence that can be derived from the resulting RWE. It is important to note that a dataset that is appropriate for one purpose may not be suitable for other evaluations. Although real-world evidence (RWE) has the potential to provide new and comprehensive insights into the safety and effectiveness of medical products, it is crucial to evaluate the relevance and quality of the data to establish claims of causal inference regarding treatment effects and internal validity of study findings, especially in non-randomized studies.
Objective metrics like accuracy, validity, and completeness should be employed to assess data quality, along with information on the original data collection methods and any subsequent modifications. This comprehensive understanding of the data’s strengths and limitations is essential for interpreting the results accurately.
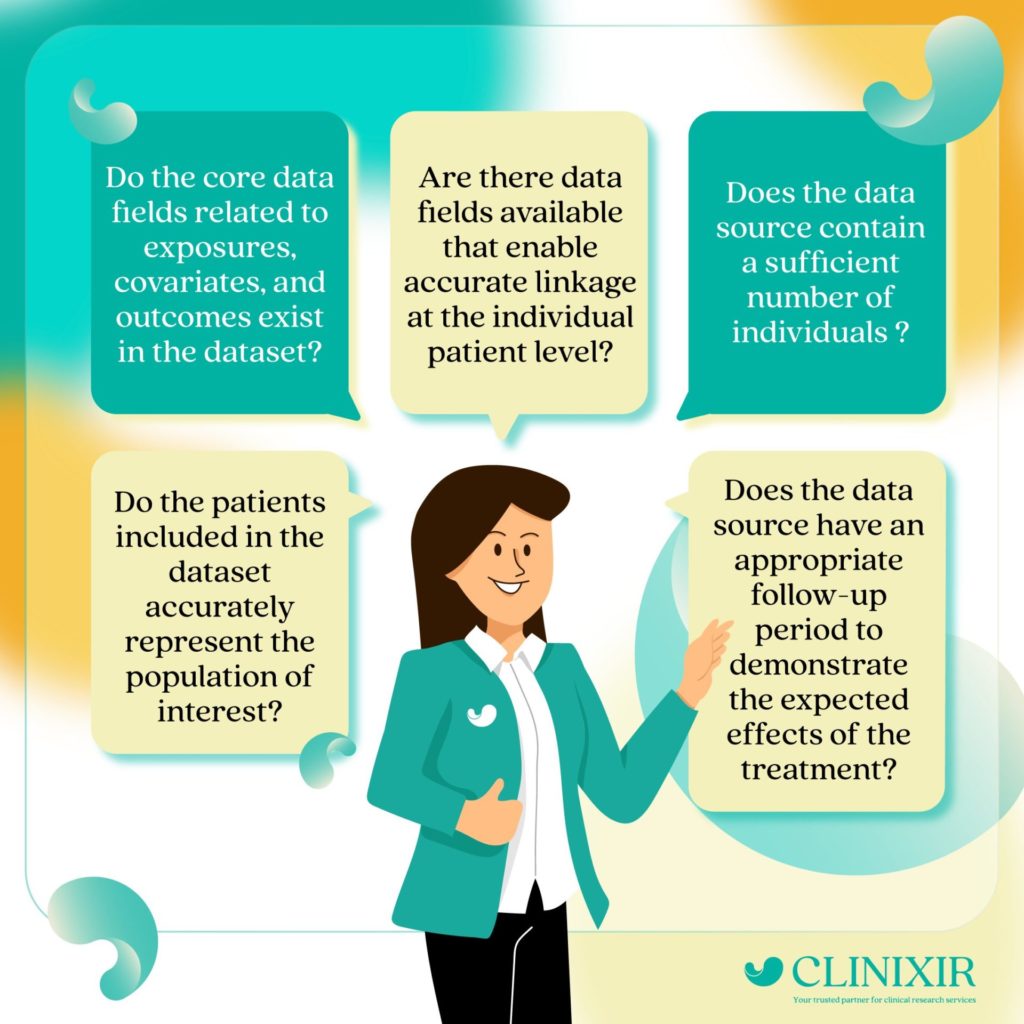
Understanding ‘Data Relevancy’
To determine if a real-world dataset is fit for purpose in relation to a particular disease or therapeutic area, it should be assessed based on its data relevancy and data quality. Data relevancy entails examining whether the dataset is comprehensive and representative of the relevant population. This evaluation focuses on whether the dataset can effectively address the regulatory question within the relevant clinical context, independent of the data quality assessment. When assessing these dimensions, special attention is given to potential selection bias.
Understanding ‘Data Quality’
Data quality dimensions encompass the precision, comprehensiveness, and transparency of real-world data (RWD) and aim to address potential information bias that might affect internal validity.
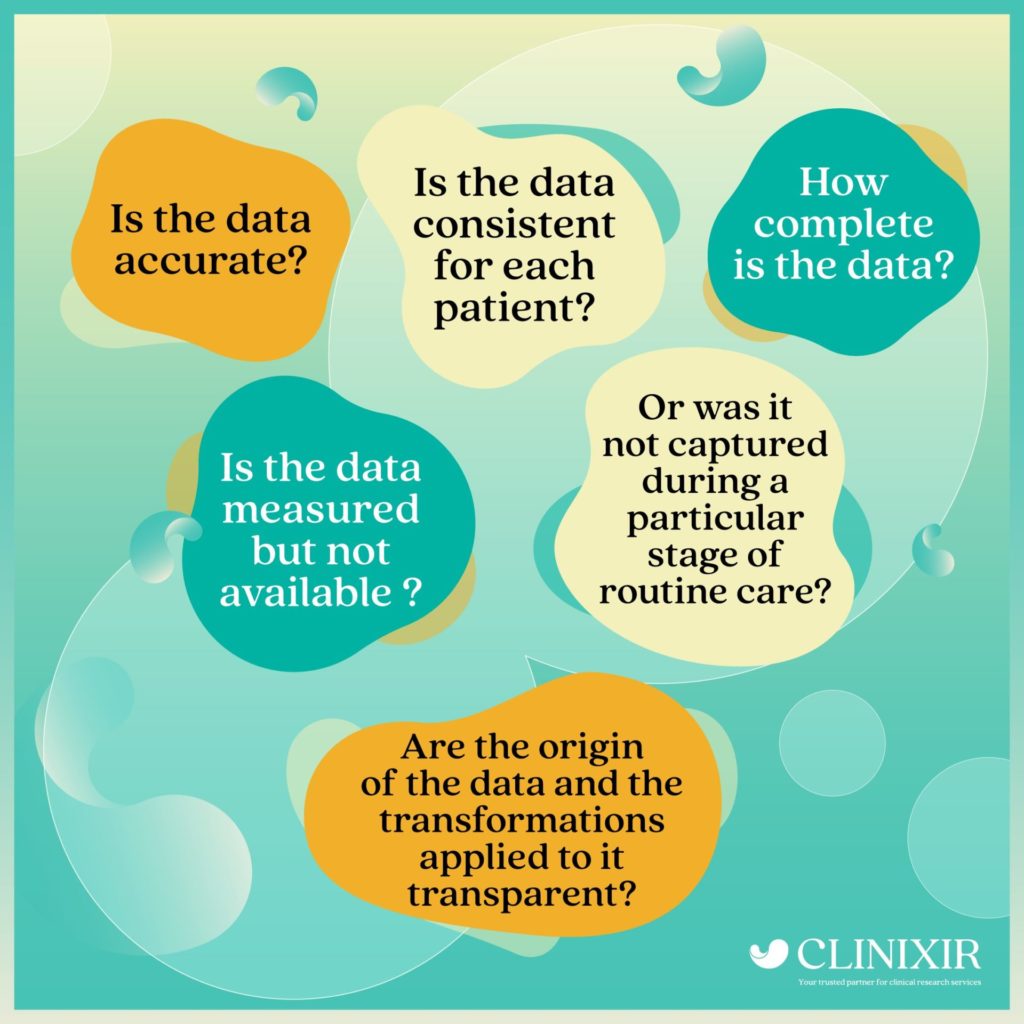
In considering these key questions, it enables the assessment of crucial exposure and outcome variables in relation to the source data. In cases where the data is found in more than one field, this may involve specifying the exact location within the record from which the data was extracted, rather than just indicating which record it is in.
Processing Raw RWD into ‘Fit For Regulatory Purpose’ RWD
The process of creating a real-world dataset that is ‘fit-for-purpose’ starts with the selection of a data source. Common examples of raw RWD sources include electronic health records. Emerging sources such as bioinformatics and public health databases among other evolving technologies are also recognized in the industry. These sources of RWD contribute to our understanding of potential risks, exposures, and health outcomes. Furthermore, they can be used for regulatory decision-making. It is important to note that each varying form and source of RWD presents distinct advantages and limitations in terms of quality and relevancy.
Real-world data (RWD) has the potential to provide valuable insights into patient health and can guide regulatory decisions. The FDA has made significant progress in making use of RWD for post-market safety surveillance and intends to further explore its application for regulatory decisions related to effectiveness. Achieving this goal necessitates a clear understanding of when and how real-world evidence (RWE) is suitable for various regulatory purposes, extending beyond safety considerations.
Main Source:
https://healthpolicy.duke.edu/sites/default/files/2020-03/characterizing_rwd.pdf


