Clinical trials play a crucial role in the emergence of new drugs, medical devices, and treatments. Oftentimes, conventional methods of on-site monitoring can cause major delays in the overall progress and time taken to complete these trials. In other cases, traditional methods have proven to be restrictive and fail to comply with regulatory standards. Taking all of these challenges into consideration, a new and modern method known as Remote Patient Monitoring (RPM) has emerged, proving to be more effective and time efficient.
Healthcare providers (HCPs) and Contract Research Organisation’s (CROs) are moving towards this modern and innovative approach to clinical trials. In doing so, the process of clinical trials is streamlined.
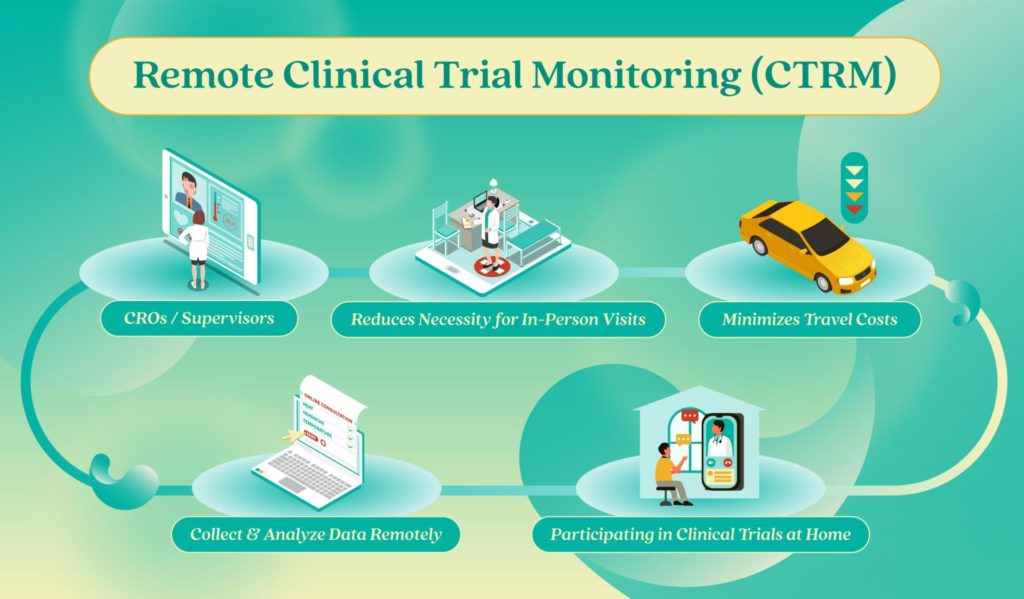
In essence, Remote Clinical Trial Monitoring (or CTRM) allows for sponsors to collect and analyze the data remotely. This approach reduces the necessity for in-person visits and minimizes travel costs for participants. It places emphasis on a patient-centric viewpoint, allowing individuals to participate in clinical trials comfortably from their own homes.
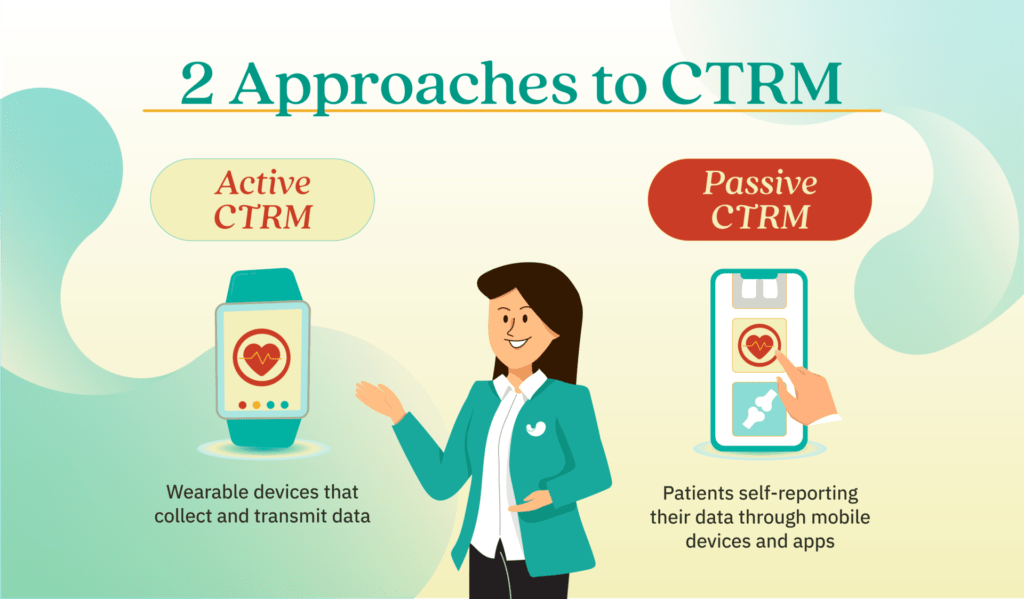
Each approach has its own advantages and disadvantages, and the decision depends heavily on the distinct requirements of each individual clinical trial.
Benefits of Remote Monitoring
- Cost Savings
CTRM allows for CROs (relevant parties) to reduce overall trial-related expenditures; i. e., the elimination of extensive travel, reduction in site visits, and efficient utilization of clinical trial personnel.
- Accelerated Trial Timelines
CTRM enables the advancement of clinical trial schedules through various means; that is, it expedites the recruitment of patients, streamlines the gathering of data, and lessens the necessity for frequent site visits, ultimately speeding up the entire trial process.
- Groundbreaking Treatments & Medical Advancements
In addition to cost and time effectiveness, CTRM plays a crucial role in propelling the exploration of new treatments. In doing so, CTRM enhances patient safety, and elevates data quality–thus, resulting in groundbreaking treatments and medical advancements.
By leveraging the power of Clinical Trials Remote Monitoring, sponsors are able to efficiently and cost effectively initiate studies. Key considerations include cost savings, streamlined processes which in turn result in speedier discoveries of groundbreaking treatments.
Partner with Clinixir today to fully leverage the advantages of clinical trial remote monitoring (CTRM).
Clinixir is a leading contract research organization in Thailand, providing full-service clinical research solutions for medical innovations. For more information contact Clinixir at enquiries@clinixir.com or https://www.clinixir.com/contact-us/. To learn more about Clinixir’s services, please visit www.clinixir.com