Within the context of the Clinical Data Management (CDM) framework, data integration involves consolidating diverse sources of clinical research data into an Electronic Data Capture (EDC) system. The ultimate goal is to present the data on the EDC interface.
Executing CDM data integration entails tasks such as performing EDC backend programming, validating programming, and maintaining ongoing data connections.
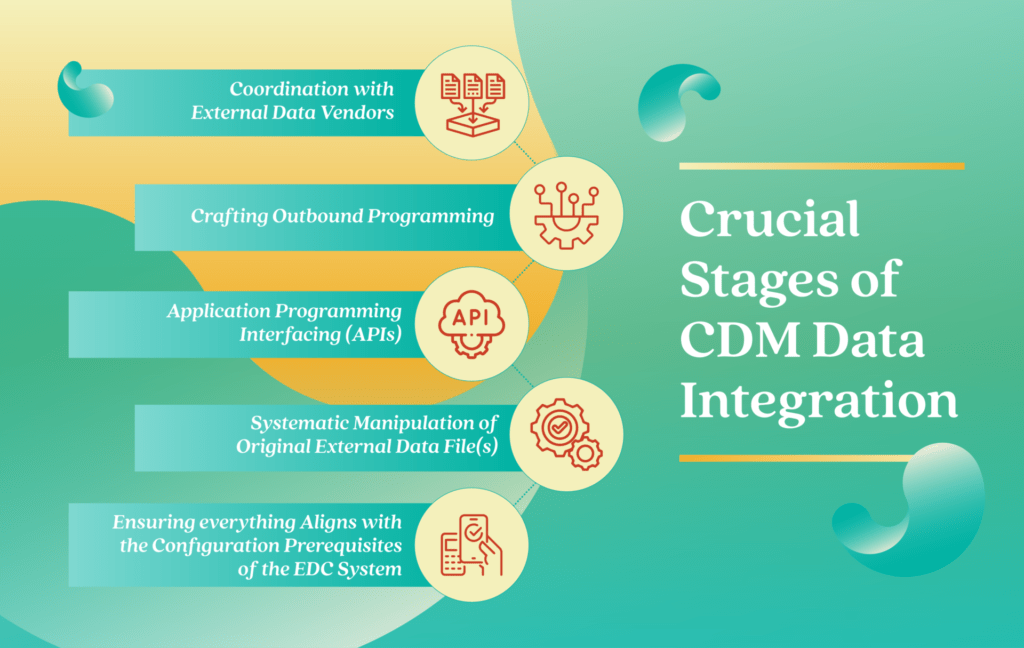
This process involves coordination with external data vendors, who must possess the technical proficiency to support EDC backend programming. The process also entails crafting outbound programming that facilitates the linkage between different data systems through web services or Application Programming Interfaces (APIs).
Additionally, this endeavor demands systematic manipulation of the original external data file to align it with the configuration prerequisites of the EDC system. This procedure can be intricate and requires careful handling.
It is important to note that any form of data manipulation during this process has the potential to diminish the integrity of the initial data set. Even with validation, the process of reconfiguring these files could introduce human errors into the programming code, subsequently impacting the dataset. Therefore, it is imperative that this process is executed with meticulous care and attention to detail, ensuring that the data remains accurate and trustworthy for its intended purpose.