Introduction
In the realm of clinical trials and research studies, a contemporary method known as electronic consent, or eConsent, has emerged to revolutionize the way informed consent is obtained from participants. Steering away from the conventional paper-based approach, eConsent leverages digital platforms or software to present participants with essential information and enable them to provide their consent electronically, often through online interfaces.
Embracing this innovative technique empowers researchers to streamline the consent process, elevating efficiency and efficacy while fostering heightened participant engagement and superior data quality in their studies.
What to Consider in an eConsent Platform
When selecting an eConsent platform for clinical research, it’s crucial to look beyond basic form creation and signing capabilities. Not all software offers the necessary compliance and workflow features that research sites require.
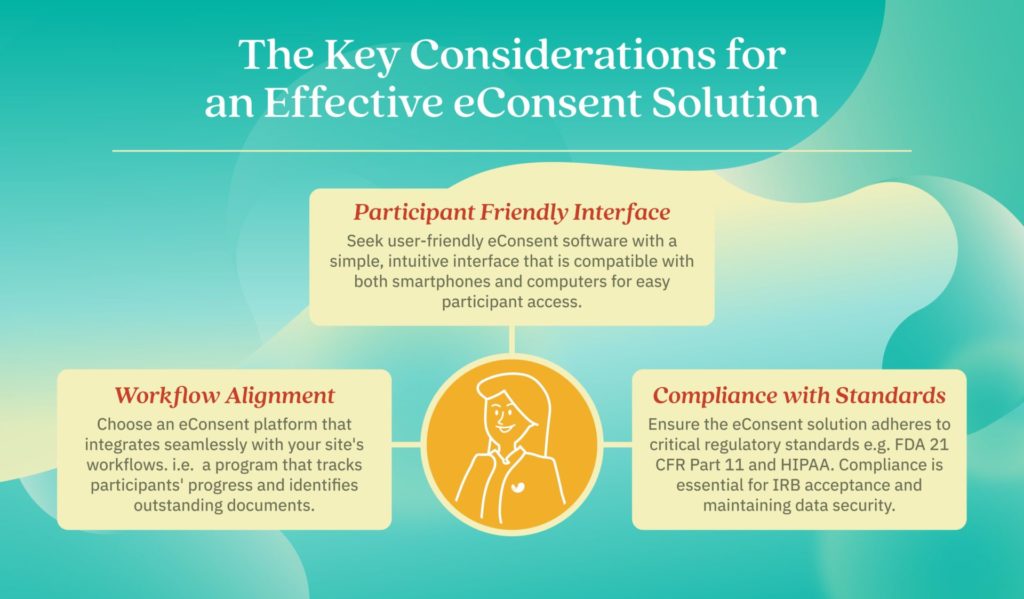
By carefully evaluating eConsent platforms based on overall usability, workflow integration, and adherence to regulatory standards, research sites can select a solution that meets their unique needs and ensures a seamless and compliant eConsent process. Thus, enabling efficient compliance oversight and minimizing study delays.
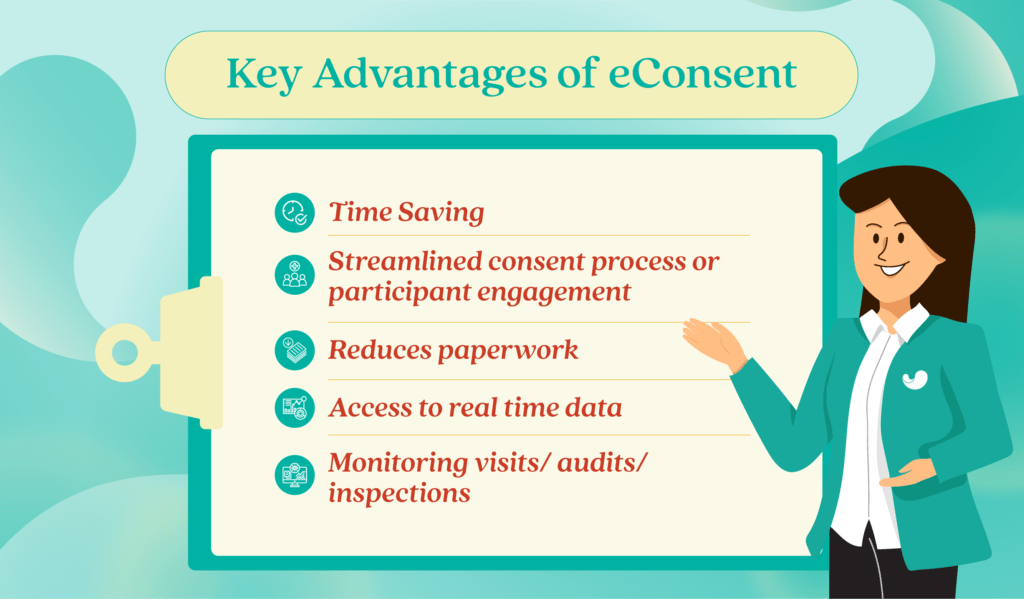
Key Advantages of eConsent
- The time-saving and logistical advantages of eConsent benefit patients, investigators, clinical trial sites, and sponsors.
- eConsent streamlines consent processes, leading to improved participant engagement.
- eConsent reduces paperwork for clinical staff, enhancing accuracy and efficiency.
- Sponsors gain real-time participant consent data, supporting study management and regulatory processes.
- eConsent helps cut down on paperwork during monitoring visits, audits, and inspections.
Conclusion
In today’s rapidly evolving landscape for healthcare, embracing digital solutions has become paramount for achieving success. eConsent forms represent a revolutionary shift in the consent process, transforming how participants interact with and provide informed consent for research studies.
At Clinixir, our adoption of eConsent forms showcases our dedication to harnessing cutting-edge technologies to drive progress in clinical research. By staying at the forefront of digital innovation, Clinixir establishes itself as an industry leader, attracting top talent, forging strategic partnerships, and delivering exceptional outcomes for our clients and patients.
Clinixir is a leading contract research organization in Thailand, providing full-service clinical research solutions for medical innovations. For more information contact Clinixir at enquiries@clinixir.com or https://www.clinixir.com/contact-us/. To learn more about Clinixir’s services, please visit www.clinixir.com